WP10 - Ethics
Objectives
The objectives of this work package are to ensure that all appropriate ethical and regulatory (legal) principles are established and applied across the entire project. Deliverables are compliances with all relevant acts and guidelines. The SysmedIBD project will:
- Ensure that best-practice for research integrity is followed across the project
- Fulfil ethical and regulatory requirements for animal experimentation
- Fulfil ethical requirements for maintaining patient confidentiality
- Establish protocols for protecting sample identity and data security
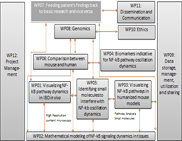
Workpackage Description
Statement of ethical principle to be followed
Research within SysmedIBD will comply with the highest ethical standards, including those of FP7. A good balance between the research objectives and the means used to achieve them will be adopted throughout. All research conducted by the partners will require ethical approval through the responsible local or national ethical committees for all aspects of research work where approval for specific activities is required on legal and ethical grounds. The Ethics Advisory Board (this is the over-arching ethics committee for the project), in collaboration with the Project Management Team, will ensure that all current ethical guidelines are followed and all relevant permissions in place; tasks for which such permissions are not yet available will not be allowed to start until evidence of approval is made available (for such tasks, partners will apply for permissions in advance of Contract completion to ensure timely deliverables).
The objective of WP10 is to consider ethical implications of the experimental system used. Within this consortium, several ethical issues need to be considered, as we will be working with genetically modified animals, with mice carrying human tissues (human-mouse xenografts), and with patient materials collected within this consortium. In addition, we will use material from bio-banks. By following local ethical and legal requirements, it is essential that each partner has all necessary permissions in place (ethical, confidentiality, material transfer agreements to protect possible intellectual property) when material is exchanged between partners. It is essential to collect information on the regulation in each partner country, to put a mechanism in place to ensure that all experiments and medical protocols are properly authorised and controlled and that protocols are established to ensure that issues related to patient confidentiality and tissue use and storage and data storage meet robust criteria for safety and security. Throughout the project it is imperative that everyone involved follows the recognised best practice for research integrity.
The management of SysmedIBD will ensure the best practice is followed in all aspects of the programme. All relevant work will be carried out according to national and international regulations concerning work with:
- Genetically modified organisms
- Laboratory animal
- Human tissues and samples
- Patient participation and confidentiality
Research integrity
Best practice for experimental design, training and research ethics will be followed throughout, as detailed in The European code of conduct for research integrity (www.allea.org March 2011). Systematic research protocols will be used with defined procedures for collection and storage of research data and related meta data. During the first 6 months of the project the project Ethics Committee will in consultation with all partners develop a Ethical Guidelines report to define best-practice that will be followed throughout the project. This will document all ethical and practical issues related to research integrity and research procedure, ethical procedures related to animal experimentation and ethical procedures related to working with patient and the use and storage of clinical samples.
Animal studies
For animal experimentation each participant will operate under agreements by each country's relevant administration. The project management plan fully incorporates consideration of ethical and safety issues, and will ensure that each partner has the required understanding, and that this knowledge is regularly updated via liaison with relevant agencies of each Institute.
All work will adhere in general to the charter of fundamental rights of the European Union (2000/c 364/01) and follow the following laws and code: * European Law :Directive 86/609EEC; * Council directive of 24 November 1986 on the approximation of laws, regulations and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes (86/609/EEC); * Opinion of the European group on ethics in science and new technologies to the European Commission, number 7, 21.6.86; * European Law : Directive 90/219EEC; * Council directive of 23 April 1990 on the contained use of genetically modified micro-organisms (90/219/EEC). In accordance with the Amsterdam protocol on animal protection and welfare, animal models will only be used when suitable alternatives cannot be found. Animal experiments will be undertaken with the highest standard of animal welfare. Experimental design for transgenic mouse models will be performed following the EU directives of the European Group on ethics relevant to genetic modification of animals, under supervision of local Ethical Committees of each host Institution.
Patient participation and confidentiality
The terms and conditions under which patients contribute to the research undertaken in this project will be clearly defined under the conditions of participation. National guideline on establishing and maintaining patient confidentially will be followed. Clear protocols for collecting and storing patient meta-data samples will be established and procedures for maintain confidentiality defined. Local ethical and legal guidelines will be followed in relevant host institutions and copies of all relevant documentation sent to the management team. Any project specific requirements will be reviewed at regular (6 monthly) intervals.